Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of cardiometabolic syndrome, which often also includes obesity, diabetes, and dyslipidemia. It is rapidly becoming the most prevalent liver disease worldwide. A sizable minority of NAFLD patients develop nonalcoholic steatohepatitis (NASH), which is characterized by inflammatory changes that can lead to progressive liver damage, cirrhosis, and hepatocellular carcinoma. Recent studies have shown that in addition to genetic predisposition and diet, the gut microbiota affects hepatic carbohydrate and lipid metabolism as well as influences the balance between pro-inflammatory and anti-inflammatory effectors in the liver, thereby impacting NAFLD and its progression to NASH. Several studies implicate the involvement of the gut microbiome with NASH or NAFLD in mice and humans. High-fat diet-fed germ-free mice exhibit lower levels of lipids in the liver in comparison with the conventionally housed mice (Rabot et al, 2010). Microbiome transferred into germ free mice, from mice that developed fasting hyperglycemia and insulinemia, but not from healthy mice, led to the development of NAFLD in recipient mice (Le Roy et al, 2013). Using 16S rDNA profiling of mice, two bacterial species, Lachnospiraceae bacterium 609 and Barnesiella intestinihominis, were found to be significantly overrepresented in the stool with a potency to induce NAFLD, while Bacteroides vulgatuswas underrepresented in comparison with the control (Le Roy et al, 2013). Furthermore, a correlation analysis of NAFLD-associated parameters and the abundance of bacterial species in mice fed with low-fat and high-fat diet showed association between Lactobacillus gasseriand Lactobacillus taiwanensisand the area of lipidic droplets in the liver (Zeng et al, 2013). The decisive role of microbiota in NAFLD development is further confirmed by a study where differences in microbiota composition between the NAFLD-resistant and NAFLD- susceptible phenotypes were identified (Le Roy et al, 2013). In this study, only germ-free mice receiving NAFLD-prone microbiota developed hyperglycemia, hyperinsulinemia, and hepatic steatosis, indicating that the tendency to develop NAFLD is transmissible through gut microbiota transplantation. Further exciting evidence comes from recent studies using human donors.
Gut microbiota from obese humans induced the onset of hepatic steatosis through modulation of lipid metabolism transcriptional profiles in germ-free mice. Furthermore, mice receiving microbiota from the same donor but after weight loss exhibit normal liver physiology (Wang et al, 2018). Moreover, some species in humans were associated with NAFLD, with the abundance of bacterial species, such as Proteobacteria, Enterobacteria, and Escherichia (Zhu et al, 2013), or Bacteroides (Boursier et al, 2016), being higher in patients with NASH as compared to matched healthy individuals. Collectively, these association studies show that correlations may exist between bacterial composition and distinct taxa and NAFLD or NASH. Several potential mechanisms by which gut microbiota regulates NAFLD and NASH have been studied in recent years. Suggested mechanisms include dysbiotic bacteria and their derived products translocating to the liver through a disrupted gut barrier, where they evoke a hepatic inflammatory reaction and commensal or metabolite-induced interplays with dietary factors in inducing steatosis. The concept of gut bacteria having influence on liver homeostasis stems from the intimate anatomical interaction between the gastrointestinal tract and the liver, which is often termed “gut–liver axis”. Disturbance of the gut–liver axis was shown to play a pivotal role in the pathogenesis of NAFLD. These include gut barrier disruption, bacterial translocation and inflammatory response in the liver, such as Toll-like receptor (TLR) signaling and inflammasome activation, and changes in composition of bacterial products.
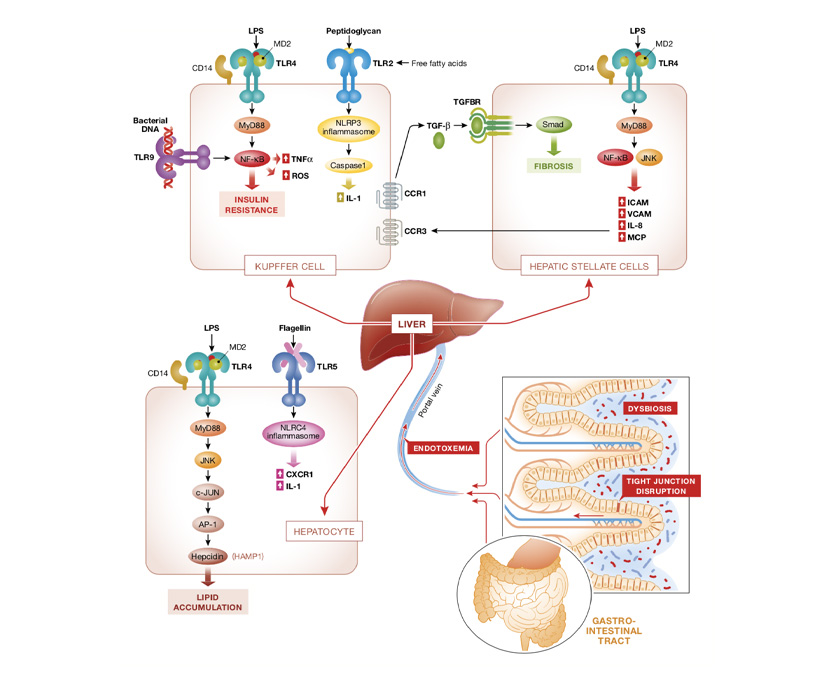
The healthy intestinal epithelium forms a tightly sealed physical barrier that separates the host from the contents of the gut. Tight junctions are one of the most important physiological and pathological regulators of intestinal permeability. Under physiological conditions, tight junction proteins, such as zonula occludens, seal adjacent epithelial cells at the apical sites and prevent bacteria from entering the intestinal mucosa and the bloodstream (Turner, 2009). However, such regulation becomes pathological with disruption of tight junctions and excessive paracellular leakage of non-self antigens to the lamina propria, which subsequently leads to the development of NASH (Fasano, 2008). A delicate crosstalk and balance among gut microbiota, intestinal epithelial cells, and gut mucosal system are important to maintain intestinal permeability and tissue homeostasis (Peterson & Artis, 2014). There is emerging evidence for a dysfunctional gut barrier or altered gut permeability in NAFLD patients (Miele et al, 2009; Volynets et al, 2012; Luther et al, 2015). A meta-analysis based on five clinical studies shows that NAFLD and NASH patients are more likely to have altered gut permeability in comparison with healthy controls (Luther et al, 2015). The association of increased intestinal permeability is stronger particularly in NASH patients, demonstrating that the inflammatory changes observed in NASH might be caused by the increased intestinal permeability (Luther et al, 2015). On the molecular level, NAFLD patients exhibit decreased expression of zonula occludens-1 (ZO-1) and junctional adhesion molecule A (JAM-A; Miele et al, 2009; Rahman et al, 2016). Gut barrier disruption leads to the translocation of overgrowing bacteria and their products to mucosa and circulation, and hence initiates or enhances liver inflammation (Fig 2). There is already an increased bacterial translocation from the gut toward blood and remote tissues at the early onset of HFD-induced type 2 diabetes, which leads to a continuous metabolic bacteremia (Amar et al, 2011). In this context, the most extensively studied microbial molecule is LPS, a cell wall component of gram-negative bacteria, also known as endotoxin (Soares et al, 2010). Systemic LPS concentration is significantly elevated in NAFLD in both human and animal studies (Yang et al, 1997; Bergheim et al, 2008; Harte et al, 2010; Sharifnia et al, 2015). LPS, a pathogen-associated molecular pattern (PAMP), is recognized by the specific pattern recognition receptor, called Toll-like receptor 4 (TLR4) and its co-receptors, LPS binding protein (LBP) and CD14. Activation of TLR4 triggers downstream inflammatory cascade, involving, depending on the context, NF-KB, AP-1, and IRF3 activation (Zhai et al, 2004; Abu-Shanab & Quigley, 2010) .in addition to TLR4, other TLRs are also found to play role in the development of NASH, including TLR9, TLR5, and TLR2.
The targeting of gut microbiota as a noninvasive diagnostic tool that correlates with the severity of NAFLD/NASH holds promise as a future therapeutic modality in these currently cureless disorders. Initial studies suggest that the progression of NAFLD and NASH may be monitored microbiome composition (Loomba et al, 2017). The administration of Postbiotics, butyrate, effectively ameliorates lipid accumulation and liver inflammation in animal NAFLD models, through modulation of gut microbiota and gut barrier function (Zhou et al, 2017b), attenuation of inducible nitric oxide synthase (iNOS) induction (Jin et al, 2016), and suppression of inflammatory pathways (Sunet al, 2018). Although most SCFAs are also potent signaling molecules through binding to G-protein-coupled receptors GPR41, GPR43, and GPR109A (Samuel et al, 2008; Maslowski et al, 2009). One of the mechanisms by which short-chain fatty acids affect fat accumulation both in the liver and in the adipose tissue is regulation of insulin sensitivity via GPR43. Short-chain fatty acid activation of GPR43 signaling in adipose tissue promotes energy expenditure and inhibits fat accumulation in adipose tissue as well as in the liver (Kimura et al, 2013). The implication of gut microbiome-based precision medicine, Postbiotics interventions, might also be an important consideration for NAFLD treatment (Thaiss et al, 2016).
Text and Image re-adapted from “The role of the microbiome in NAFLD and NASH”